Exploring the Periodic Table: A Deep Dive into Advanced Trends and Their Impact
Related Articles: Exploring the Periodic Table: A Deep Dive into Advanced Trends and Their Impact
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Exploring the Periodic Table: A Deep Dive into Advanced Trends and Their Impact. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Exploring the Periodic Table: A Deep Dive into Advanced Trends and Their Impact
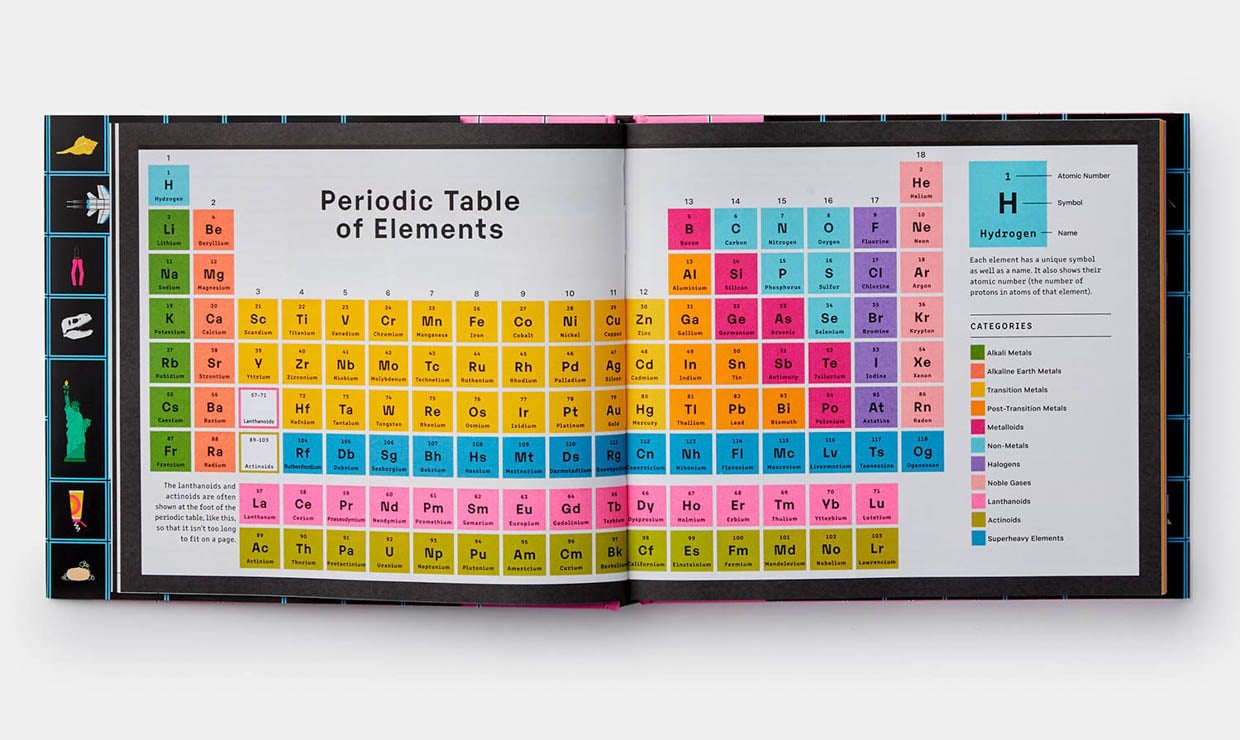
The periodic table, a cornerstone of chemistry, is a powerful tool that organizes the elements based on their properties and reveals fascinating patterns in their behavior. While basic periodic trends like electronegativity and ionization energy are well-established, a deeper understanding of advanced periodic trends is crucial for navigating complex chemical interactions and predicting the behavior of elements in diverse contexts.
Advanced Periodic Trends are those that go beyond the fundamental trends and delve into more nuanced aspects of element behavior. These trends often involve the interplay of multiple factors, including electron configuration, atomic size, and nuclear charge, and they can be influenced by external conditions like pressure and temperature. Understanding these advanced trends is essential for researchers in fields like materials science, catalysis, and drug development.
POGIL (Process Oriented Guided Inquiry Learning) is a pedagogical approach that emphasizes active learning and collaborative problem-solving. It encourages students to develop critical thinking skills and a deeper understanding of scientific concepts through guided inquiry. POGIL 2025 represents a forward-looking vision of POGIL, incorporating cutting-edge research and incorporating the latest advancements in scientific knowledge.
The Importance of Advanced Periodic Trends in POGIL 2025
In the context of POGIL 2025, advanced periodic trends play a pivotal role in enhancing student learning and fostering a deeper understanding of chemistry. By incorporating these trends into the curriculum, students gain a more comprehensive and nuanced perspective on the behavior of elements, leading to:
- Enhanced Prediction and Interpretation: Students can better predict and interpret chemical reactions and properties based on the interplay of multiple periodic trends.
- Improved Problem-Solving Skills: The complexities of advanced trends encourage students to develop critical thinking skills and analytical approaches to problem-solving.
- Increased Engagement and Curiosity: The intricate nature of advanced trends sparks curiosity and encourages students to explore the fascinating world of chemistry beyond basic concepts.
- Relevance to Real-World Applications: The study of advanced trends directly connects to real-world applications in various fields, making chemistry more engaging and relevant to students’ lives.
Exploring Advanced Periodic Trends in Depth
Let’s delve into some key advanced periodic trends that are crucial for understanding the behavior of elements:
1. Relativistic Effects:
These effects arise from the very high speeds of electrons in heavier atoms, approaching the speed of light. Relativity dictates that mass increases with velocity, leading to a contraction of the s and p orbitals, especially in elements with high atomic numbers. This contraction, in turn, influences atomic size, ionization energy, and other properties.
- Example: The unexpectedly high melting point of gold, despite its relatively low ionization energy, is attributed to relativistic effects.
2. Lanthanide Contraction:
This phenomenon refers to the gradual decrease in atomic radii across the lanthanide series (elements 57-71). The poor shielding effect of the 4f electrons leads to a stronger attraction between the nucleus and the outer electrons, resulting in smaller atomic sizes.
- Example: The lanthanide contraction explains the similarities in ionic radii and chemical properties between elements in the 4d and 5d transition series.
3. Diagonal Relationships:
These relationships occur between elements diagonally positioned in the periodic table, like lithium and magnesium or beryllium and aluminum. Due to similar ionic radii and electronegativity, these elements exhibit similar chemical properties.
- Example: Lithium and magnesium both form strong ionic bonds with oxygen, resulting in the formation of oxides with similar properties.
4. Inert Pair Effect:
This effect explains the tendency of heavier elements in groups 13-16 to exhibit a +2 oxidation state, despite having the potential to achieve a higher oxidation state. The reluctance of the ns² electrons to participate in bonding is attributed to the inert pair effect.
- Example: Lead, a heavier element in group 14, commonly forms Pb²⁺ ions, while tin can exhibit both +2 and +4 oxidation states.
5. Periodic Trends in Reactivity:
Reactivity is a complex function of multiple factors, including ionization energy, electron affinity, and electronegativity. The periodic trends in these properties influence the reactivity of elements.
- Example: The alkali metals (group 1) are highly reactive due to their low ionization energies and tendency to lose electrons easily.
6. Periodic Trends in Bonding:
The type of chemical bond formed between elements is influenced by the electronegativity difference between them. Elements with similar electronegativities form covalent bonds, while elements with large electronegativity differences form ionic bonds.
- Example: Sodium and chlorine form an ionic bond, while carbon and hydrogen form a covalent bond.
7. Periodic Trends in Physical Properties:
The periodic table reveals predictable patterns in physical properties like melting point, boiling point, and density. These trends are often related to atomic size, bonding strength, and intermolecular forces.
- Example: The melting point generally increases across a period due to stronger metallic bonding and decreases down a group due to weaker interatomic forces.
8. Periodic Trends in Spectroscopic Properties:
The electronic configurations of elements influence their spectroscopic properties, including absorption and emission spectra. These properties are often used for analytical purposes.
- Example: The characteristic yellow color of sodium flame tests is attributed to the emission of light at a specific wavelength when sodium atoms are excited.
Related Searches and FAQs
1. Periodic Trends and Chemical Reactions:
- Q: How do periodic trends influence the reactivity of elements in chemical reactions?
- A: Periodic trends influence the reactivity of elements by determining their tendency to lose or gain electrons. For example, elements with low ionization energies readily lose electrons and are thus highly reactive, while elements with high electron affinities readily gain electrons and are also highly reactive.
2. Applications of Periodic Trends in Materials Science:
- Q: How are periodic trends utilized in the development of new materials?
- A: Periodic trends provide insights into the bonding properties, electrical conductivity, and other characteristics of elements, guiding the design of materials with specific properties. For example, the high melting point of tungsten, a consequence of its strong metallic bonding, makes it suitable for use in light bulbs.
3. Periodic Trends in Catalysis:
- Q: What role do periodic trends play in catalysis?
- A: Understanding periodic trends allows scientists to select catalysts with specific properties, influencing reaction rates and selectivity. For example, transition metals with variable oxidation states are often used as catalysts due to their ability to facilitate electron transfer during reactions.
4. Periodic Trends in Drug Development:
- Q: How are periodic trends utilized in the design of new drugs?
- A: Periodic trends guide the selection of elements for incorporating into drug molecules, influencing their properties like solubility, stability, and bioactivity. For example, the use of fluorine in drug development is often influenced by its electronegativity and ability to enhance lipophilicity.
5. Advanced Periodic Trends and the POGIL Approach:
- Q: How does the POGIL approach incorporate advanced periodic trends?
- A: POGIL 2025 emphasizes active learning and problem-solving, incorporating advanced periodic trends into challenging scenarios and real-world applications. Students are encouraged to explore these trends through collaborative activities, fostering a deeper understanding of the subject.
6. The Future of Periodic Trends in Chemistry:
- Q: What are the future directions for research in advanced periodic trends?
- A: Future research in advanced periodic trends will focus on exploring the effects of extreme conditions like high pressure and temperature on element behavior, as well as developing new theoretical models to predict and understand these complex phenomena.
Tips for Understanding Advanced Periodic Trends
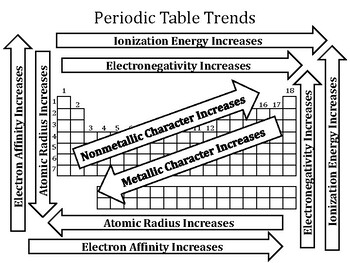
- Visualize: Utilize periodic tables with color-coded trends to visualize the patterns and relationships between elements.
- Connect to Real-World Examples: Relate the concepts to real-world applications in fields like materials science, medicine, and technology.
- Practice Problem Solving: Engage in guided inquiry activities and problem-solving exercises to apply your knowledge of advanced periodic trends.
- Explore Resources: Utilize online resources, textbooks, and research articles to deepen your understanding of advanced periodic trends.
Conclusion
Advanced periodic trends are a critical component of understanding the fascinating world of chemistry. By incorporating these trends into the curriculum, the POGIL 2025 approach empowers students to develop a comprehensive and nuanced understanding of the behavior of elements. This knowledge is essential for tackling complex chemical problems and advancing scientific understanding across various fields. As research continues to unravel the intricacies of element behavior, our knowledge of advanced periodic trends will continue to evolve, leading to new discoveries and innovations that shape our world.





Closure
Thus, we hope this article has provided valuable insights into Exploring the Periodic Table: A Deep Dive into Advanced Trends and Their Impact. We thank you for taking the time to read this article. See you in our next article!