Mastering the Periodic Table: A Comprehensive Guide for MCAT 2025 Success
Related Articles: Mastering the Periodic Table: A Comprehensive Guide for MCAT 2025 Success
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Mastering the Periodic Table: A Comprehensive Guide for MCAT 2025 Success. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Mastering the Periodic Table: A Comprehensive Guide for MCAT 2025 Success
The MCAT, a challenging standardized test for medical school applicants, evaluates a wide range of scientific knowledge, including a deep understanding of chemistry. Within this domain, periodic table trends are crucial, as they provide a framework for predicting and explaining the properties of elements and their behavior in chemical reactions. This guide will delve into the intricacies of these trends, equipping you with the knowledge and strategies necessary to conquer this section of the MCAT.
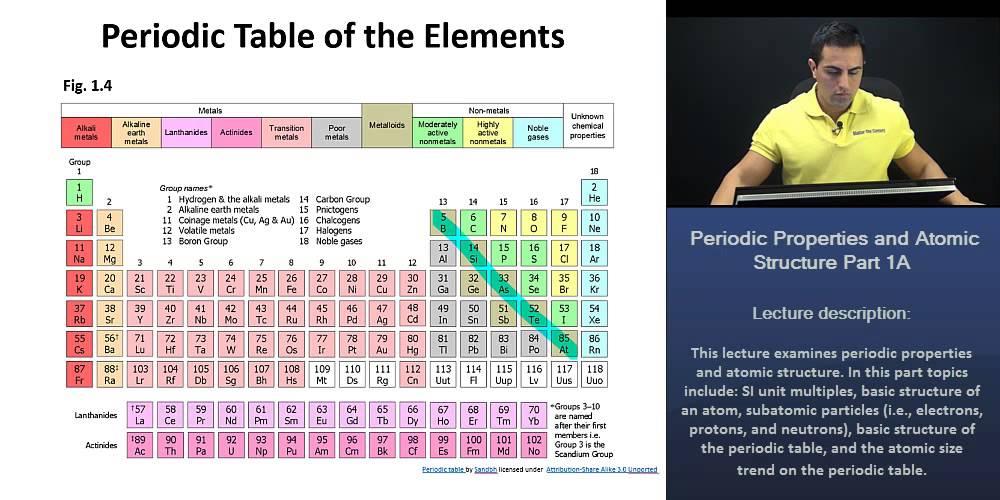
Understanding the Periodic Table’s Structure
The periodic table is not just a random arrangement of elements; it is a carefully crafted system that reflects fundamental relationships between atoms. The table is organized based on increasing atomic number, which represents the number of protons in an atom’s nucleus.
- Periods: Horizontal rows on the periodic table are called periods. Elements within a period share the same number of electron shells, meaning they have the same number of energy levels where electrons reside.
- Groups: Vertical columns on the periodic table are called groups. Elements within a group share the same number of valence electrons, which are the electrons in the outermost shell and participate in chemical bonding.
Key Periodic Table Trends
The periodic table’s structure enables us to observe predictable patterns in the properties of elements. These trends are essential for understanding chemical reactions and predicting the behavior of elements:
1. Atomic Radius: The atomic radius is the distance between the nucleus of an atom and its outermost electron shell.
- Trend: Atomic radius generally increases as you move down a group and decreases as you move across a period.
- Explanation: Moving down a group, the number of electron shells increases, pushing the outermost electrons further from the nucleus. Moving across a period, the number of protons in the nucleus increases, pulling the electrons closer to the nucleus, despite the addition of electrons to the same shell.
2. Ionization Energy: Ionization energy is the minimum energy required to remove an electron from a gaseous atom in its ground state.
- Trend: Ionization energy generally increases as you move across a period and decreases as you move down a group.
- Explanation: As you move across a period, the increasing nuclear charge attracts electrons more strongly, making it harder to remove an electron. Moving down a group, the outermost electron is further from the nucleus, experiencing weaker attraction and requiring less energy to remove.
3. Electron Affinity: Electron affinity is the change in energy when an electron is added to a neutral atom to form a negative ion.
- Trend: Electron affinity generally increases as you move across a period and decreases as you move down a group. However, there are exceptions to this trend.
- Explanation: Moving across a period, the increasing nuclear charge attracts incoming electrons more strongly, resulting in a greater energy release. Moving down a group, the incoming electron is added to a larger electron shell, experiencing weaker attraction and resulting in a smaller energy release.
4. Electronegativity: Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond.
- Trend: Electronegativity generally increases as you move across a period and decreases as you move down a group.
- Explanation: As you move across a period, the increasing nuclear charge attracts electrons more strongly, resulting in higher electronegativity. Moving down a group, the outermost electron is further from the nucleus, experiencing weaker attraction and resulting in lower electronegativity.
5. Metallic Character: Metallic character refers to the tendency of an element to lose electrons and form positive ions (cations).
- Trend: Metallic character generally increases as you move down a group and decreases as you move across a period.
- Explanation: As you move down a group, the outermost electron is further from the nucleus, making it easier to remove and exhibit metallic character. Moving across a period, the increasing nuclear charge holds electrons more tightly, reducing metallic character.
6. Reactivity: Reactivity refers to the tendency of an element to participate in chemical reactions.
- Trend: For metals, reactivity generally increases as you move down a group and decreases as you move across a period. For nonmetals, reactivity generally decreases as you move down a group and increases as you move across a period.
- Explanation: The reactivity of metals is related to their ease of losing electrons, which is influenced by metallic character. Nonmetals tend to gain electrons, and their reactivity is influenced by their electronegativity.
7. Melting Point and Boiling Point: Melting point and boiling point are the temperatures at which a substance transitions from solid to liquid and liquid to gas, respectively.
- Trend: Melting point and boiling point generally increase as you move down a group and decrease as you move across a period. However, there are exceptions to this trend.
- Explanation: Moving down a group, the larger atomic size and weaker interatomic forces lead to lower melting and boiling points. Moving across a period, the stronger interatomic forces due to increased electronegativity lead to higher melting and boiling points.
8. Shielding Effect: The shielding effect refers to the reduction in the attraction between the nucleus and valence electrons due to the presence of inner electrons.
- Trend: The shielding effect increases as you move down a group and remains relatively constant as you move across a period.
- Explanation: As you move down a group, the number of inner electron shells increases, leading to a greater shielding effect. Moving across a period, the number of inner electrons remains constant, resulting in a relatively consistent shielding effect.
Implications for MCAT 2025
Understanding these periodic table trends is crucial for success on the MCAT 2025. Here’s how these concepts are tested:
- Chemical Bonding: Knowledge of electronegativity helps predict the type of bond (ionic, covalent, polar covalent) that will form between atoms.
- Chemical Reactions: Understanding reactivity trends allows you to predict which elements are likely to participate in chemical reactions and what type of products will form.
- Acid-Base Chemistry: The periodic table trends are essential for understanding the properties of acids and bases, including their strength and reactivity.
- Organic Chemistry: Understanding the trends in electronegativity and atomic size helps explain the properties of organic molecules and their reactivity.
Related Searches
1. Periodic Table Trends and Chemical Bonding: This search explores the relationship between periodic table trends and the types of chemical bonds that form between elements. For example, understanding electronegativity helps predict whether a bond will be ionic (large difference in electronegativity) or covalent (similar electronegativity).
2. Periodic Table Trends and Reactivity: This search investigates how periodic table trends influence the reactivity of elements. For example, understanding metallic character allows you to predict the reactivity of metals, while electronegativity helps predict the reactivity of nonmetals.
3. Periodic Table Trends and Acid-Base Chemistry: This search delves into the connection between periodic table trends and the properties of acids and bases. For example, understanding electronegativity helps explain the strength of acids and bases.
4. Periodic Table Trends and Organic Chemistry: This search explores the application of periodic table trends in understanding the properties and reactivity of organic molecules. For example, understanding electronegativity and atomic size helps explain the properties of functional groups and their reactions.
5. Periodic Table Trends and MCAT 2025: This search provides resources and study materials specifically designed to help MCAT 2025 test-takers master periodic table trends.
6. Periodic Table Trends Examples: This search offers specific examples of how periodic table trends manifest in various chemical phenomena.
7. Periodic Table Trends Chart: This search leads to visual representations of periodic table trends, making it easier to visualize and remember the patterns.
8. Periodic Table Trends Quiz: This search provides quizzes and practice questions to assess your understanding of periodic table trends and prepare for the MCAT 2025.
FAQs
Q: How do I memorize all the periodic table trends?
A: Instead of rote memorization, focus on understanding the underlying reasons for the trends. Visualize the periodic table and relate the trends to the arrangement of electrons and the nuclear charge.
Q: Are there any exceptions to the periodic table trends?
A: Yes, there are exceptions to some of the trends, particularly those related to electron affinity and melting/boiling point. It’s important to be aware of these exceptions and understand the reasons behind them.
Q: How can I apply periodic table trends to solve MCAT 2025 questions?
A: Practice applying the trends to solve MCAT-style questions. Think about how the trends relate to the concepts tested in the exam, such as chemical bonding, reactions, and acid-base chemistry.
Tips for Mastering Periodic Table Trends
- Visualize: Create visual representations of the periodic table trends, using arrows and color coding to highlight the directions of change.
- Relate to Atomic Structure: Connect the trends to the underlying atomic structure, focusing on the number of electron shells, valence electrons, and nuclear charge.
- Practice with Examples: Use specific examples to reinforce your understanding of the trends. For example, compare the electronegativity of oxygen and fluorine, or the reactivity of sodium and potassium.
- Use Mnemonics: Create mnemonics or acronyms to help you remember the trends and their directions.
- Review Regularly: Periodically review the trends to ensure they are firmly ingrained in your memory.
Conclusion
Mastering periodic table trends is essential for success on the MCAT 2025. By understanding the underlying reasons for these trends and practicing their application, you can confidently tackle chemistry-related questions on the exam. Remember, the periodic table is not just a list of elements; it is a powerful tool for predicting and explaining the behavior of matter.



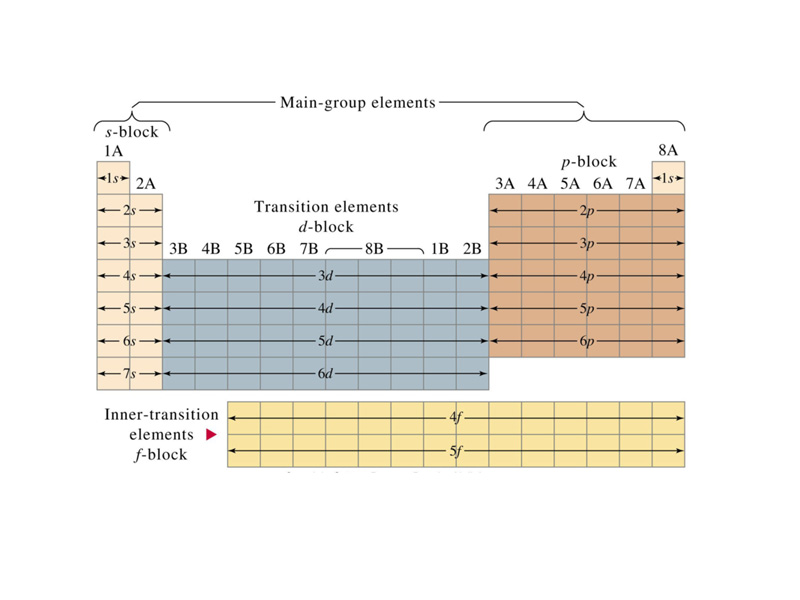

Closure
Thus, we hope this article has provided valuable insights into Mastering the Periodic Table: A Comprehensive Guide for MCAT 2025 Success. We thank you for taking the time to read this article. See you in our next article!