Navigating the Periodic Table: A Comprehensive Guide to Understanding Chemical Trends
Related Articles: Navigating the Periodic Table: A Comprehensive Guide to Understanding Chemical Trends
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Navigating the Periodic Table: A Comprehensive Guide to Understanding Chemical Trends. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Navigating the Periodic Table: A Comprehensive Guide to Understanding Chemical Trends
- 2 Introduction
- 3 Navigating the Periodic Table: A Comprehensive Guide to Understanding Chemical Trends
- 3.1 Why is Understanding Periodic Trends Important?
- 3.2 Exploring Key Periodic Trends
- 3.3 Periodic Trends Quiz 2025 – Related Searches
- 3.4 Periodic Trends Quiz 2025 – Frequently Asked Questions
- 3.5 Periodic Trends Quiz 2025 – Tips for Success
- 3.6 Periodic Trends Quiz 2025 – Conclusion
- 4 Closure
Navigating the Periodic Table: A Comprehensive Guide to Understanding Chemical Trends
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
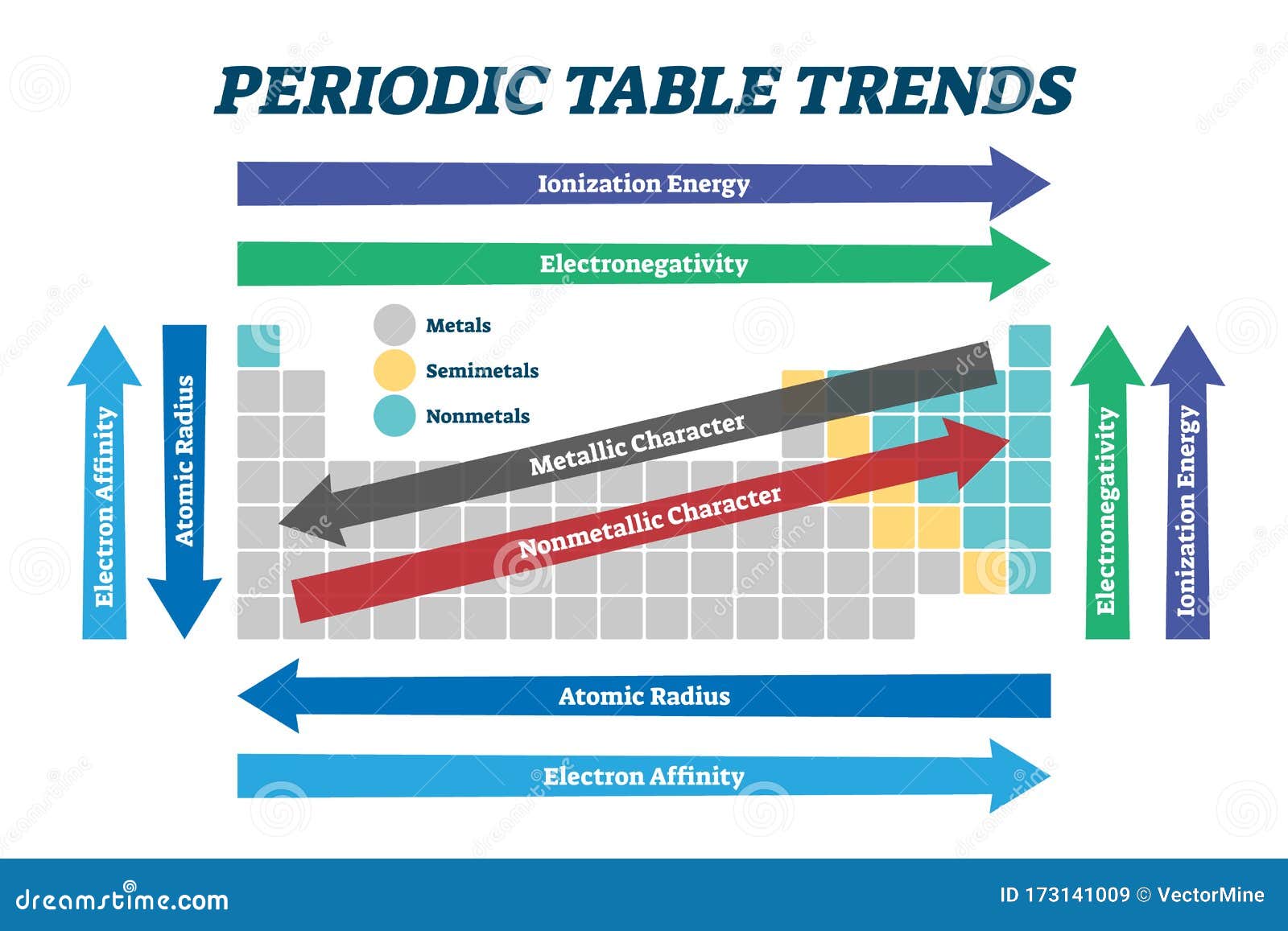
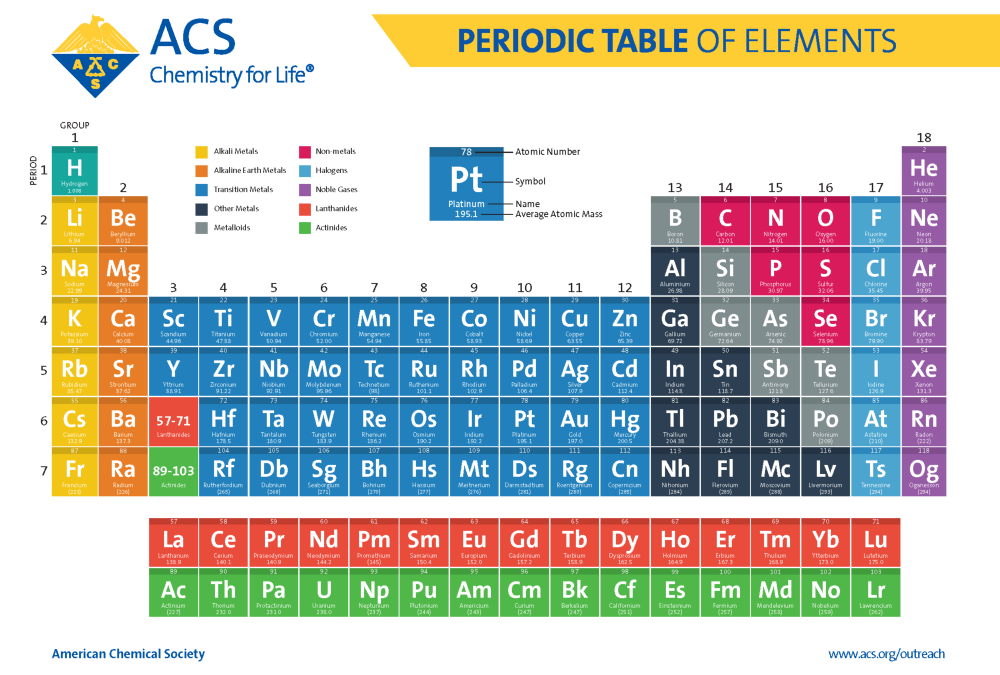
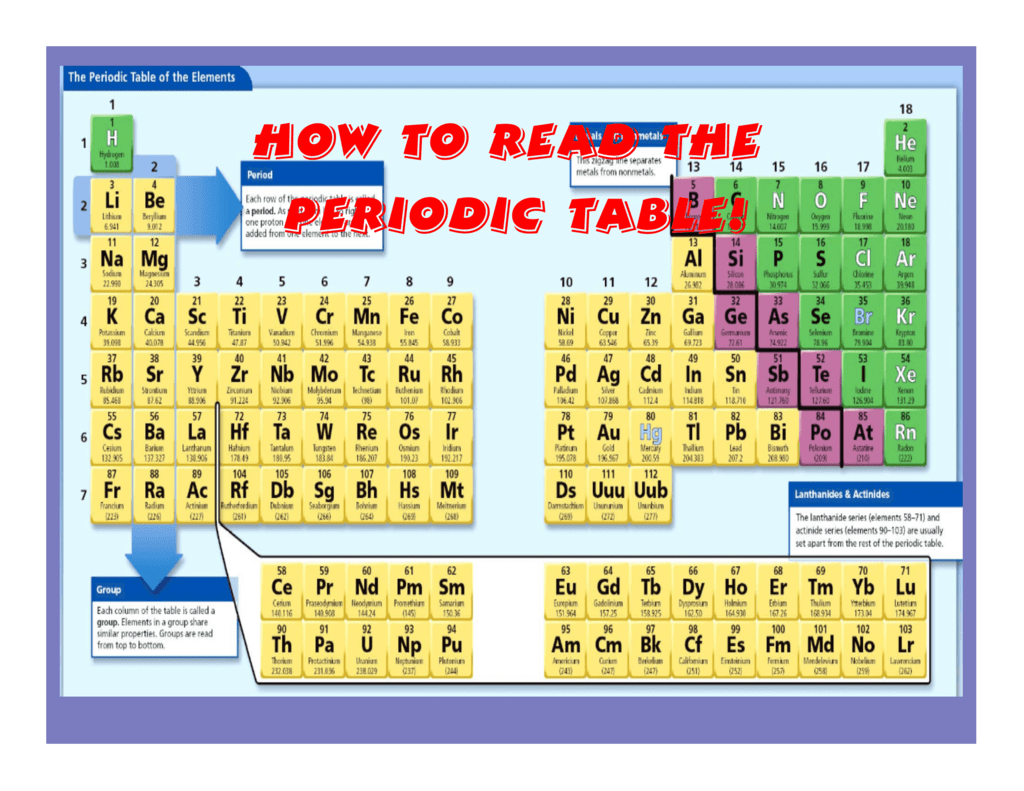
The periodic table, a cornerstone of chemistry, is a visual representation of the elements, organized by their atomic number and recurring properties. Understanding the trends within this table is crucial for predicting and explaining chemical behavior, a skill that is vital for success in chemistry studies and related fields.
Periodic Trends Quiz 2025 serves as a valuable tool for assessing and reinforcing comprehension of these trends. This quiz, designed for students and professionals alike, explores key periodic properties like atomic radius, ionization energy, electronegativity, and electron affinity. By engaging with this quiz, individuals can deepen their understanding of these concepts and their implications in chemical reactions and bonding.
Why is Understanding Periodic Trends Important?
The periodic table is not just a list of elements; it is a framework for understanding how elements interact and form compounds. The trends within the table provide valuable insights into:
- Predicting Chemical Reactions: Knowing the relative reactivity of elements based on their position on the periodic table allows chemists to predict the outcome of chemical reactions and design experiments accordingly.
- Understanding Bonding: Periodic trends influence the types of bonds formed between atoms, determining the structure and properties of molecules and compounds.
- Developing New Materials: The understanding of periodic trends guides the development of novel materials with specific properties, such as conductivity, strength, and reactivity.
- Solving Environmental Challenges: Knowledge of periodic trends is critical in addressing environmental issues like pollution and resource management.
Exploring Key Periodic Trends
The Periodic Trends Quiz 2025 focuses on eight key trends:
-
Atomic Radius: This trend describes the size of an atom, which is determined by the distance between the nucleus and the outermost electron shell. Atomic radius generally increases down a group (column) and decreases across a period (row) of the periodic table.
- Factors Influencing Atomic Radius: The number of electron shells and the effective nuclear charge (the net positive charge experienced by an electron) play a significant role in determining atomic radius.
- Applications: Atomic radius influences the packing of atoms in solids, affecting their density and physical properties.
-
Ionization Energy: This trend represents the energy required to remove an electron from a gaseous atom or ion. Ionization energy generally decreases down a group and increases across a period.
- Factors Influencing Ionization Energy: The effective nuclear charge and the distance between the nucleus and the outermost electron play a crucial role.
- Applications: Ionization energy is crucial in understanding the reactivity of elements and their tendency to lose electrons.
-
Electron Affinity: This trend measures the change in energy when an electron is added to a neutral atom in its gaseous state. Electron affinity generally increases across a period and decreases down a group.
- Factors Influencing Electron Affinity: The effective nuclear charge and the availability of empty orbitals influence electron affinity.
- Applications: Electron affinity helps predict the likelihood of an atom gaining an electron and forming an anion.
-
Electronegativity: This trend reflects the ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group.
- Factors Influencing Electronegativity: The effective nuclear charge and the distance between the nucleus and the outermost electron contribute to electronegativity.
- Applications: Electronegativity determines the polarity of chemical bonds, influencing the properties of molecules and compounds.
-
Metallic Character: This trend describes the tendency of an element to lose electrons and form cations. Metallic character generally increases down a group and decreases across a period.
- Factors Influencing Metallic Character: The number of valence electrons and the effective nuclear charge influence metallic character.
- Applications: Metallic character is crucial in understanding the properties of metals, such as conductivity and malleability.
-
Non-Metallic Character: This trend describes the tendency of an element to gain electrons and form anions. Non-metallic character generally increases across a period and decreases down a group.
- Factors Influencing Non-Metallic Character: The number of valence electrons and the effective nuclear charge influence non-metallic character.
- Applications: Non-metallic character is crucial in understanding the properties of nonmetals, such as their ability to form covalent bonds and their tendency to act as insulators.
-
Electronegativity Difference: This trend is a measure of the difference in electronegativity between two atoms in a bond. It helps determine the type of bond formed (ionic, covalent, or polar covalent).
- Factors Influencing Electronegativity Difference: The electronegativity of the individual atoms involved in the bond.
- Applications: Electronegativity difference is essential in understanding the polarity of molecules and their interactions.
-
Oxidation States: This trend describes the charge an atom acquires when it gains or loses electrons in a chemical reaction. Oxidation states can be positive (cations) or negative (anions).
- Factors Influencing Oxidation States: The number of valence electrons and the electronegativity of the atom.
- Applications: Oxidation states are crucial in understanding the reactivity of elements and their role in redox reactions.
Periodic Trends Quiz 2025 – Related Searches
Periodic Trends Quiz 2025 sparks curiosity and leads to further exploration of related concepts:
- Periodic Trends Practice Problems: Students often benefit from solving practice problems to solidify their understanding of periodic trends. These problems can range from simple identification of trends to more complex applications.
- Periodic Trends Worksheet: Worksheets provide structured exercises that focus on specific periodic trends. They can include multiple-choice questions, matching activities, and short-answer questions.
- Periodic Trends Chart: A visual representation of the periodic table with highlighted trends can be a valuable tool for learning and reference.
- Periodic Trends Table: This refers to the periodic table itself, which is often used in conjunction with periodic trends resources.
- Periodic Trends Notes: Comprehensive notes on periodic trends can serve as a valuable study guide and reference material.
- Periodic Trends Questions: Students often seek clarification on specific aspects of periodic trends by asking questions. These questions can range from basic definitions to more complex applications.
- Periodic Trends Examples: Illustrative examples of how periodic trends manifest in real-world scenarios can enhance understanding and make the concepts more relatable.
- Periodic Trends Study Guide: A study guide provides a structured approach to learning and reviewing periodic trends. It can include key concepts, definitions, examples, and practice problems.
Periodic Trends Quiz 2025 – Frequently Asked Questions
Q: What is the purpose of the Periodic Trends Quiz 2025?
A: The Periodic Trends Quiz 2025 aims to assess and reinforce understanding of the key trends within the periodic table. It helps students and professionals alike identify strengths and weaknesses in their knowledge of these fundamental concepts.
Q: Who should take the Periodic Trends Quiz 2025?
A: This quiz is suitable for anyone studying chemistry, including high school students, undergraduate and graduate students, and professionals working in related fields.
Q: How can I prepare for the Periodic Trends Quiz 2025?
A: Thorough review of the periodic table, its organization, and the key trends is essential. Studying notes, textbooks, and online resources can be helpful. Practice problems and worksheets can also solidify understanding.
Q: What are the benefits of taking the Periodic Trends Quiz 2025?
A: The quiz provides a structured assessment of understanding, identifies areas for improvement, and helps reinforce key concepts. It can also serve as a motivational tool for continued learning.
Q: How can I access the Periodic Trends Quiz 2025?
A: The availability of the Periodic Trends Quiz 2025 will depend on the specific educational institution or platform offering it. It may be accessible through online learning platforms, textbooks, or educational websites.
Periodic Trends Quiz 2025 – Tips for Success
- Visualize the Trends: Use a periodic table with highlighted trends to visualize the patterns and relationships between elements.
- Connect Trends to Real-World Examples: Relating periodic trends to real-world applications like the reactivity of metals or the properties of compounds can enhance understanding.
- Practice Regularly: Solve practice problems and complete worksheets to reinforce concepts and build confidence.
- Seek Clarification: Don’t hesitate to ask questions if you encounter difficulties.
- Utilize Online Resources: Explore online resources like videos, interactive simulations, and quizzes to supplement your learning.
Periodic Trends Quiz 2025 – Conclusion
The Periodic Trends Quiz 2025 provides a valuable tool for evaluating and enhancing understanding of the periodic table and its trends. By engaging with this quiz, individuals can solidify their knowledge of key concepts, identify areas for improvement, and gain a deeper appreciation for the fundamental principles that govern chemical behavior.




Closure
Thus, we hope this article has provided valuable insights into Navigating the Periodic Table: A Comprehensive Guide to Understanding Chemical Trends. We appreciate your attention to our article. See you in our next article!