Navigating the Periodic Table: Understanding Trends in Ionic Properties
Related Articles: Navigating the Periodic Table: Understanding Trends in Ionic Properties
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Navigating the Periodic Table: Understanding Trends in Ionic Properties. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Navigating the Periodic Table: Understanding Trends in Ionic Properties
- 2 Introduction
- 3 Navigating the Periodic Table: Understanding Trends in Ionic Properties
- 3.1 Understanding Ionic Trends
- 3.2 Key Trends in the Periodic Table
- 3.3 Applications of Ionic Trends
- 3.4 Related Searches
- 3.5 FAQs
- 3.6 Tips for Understanding Ionic Trends
- 3.7 Conclusion
- 4 Closure
Navigating the Periodic Table: Understanding Trends in Ionic Properties
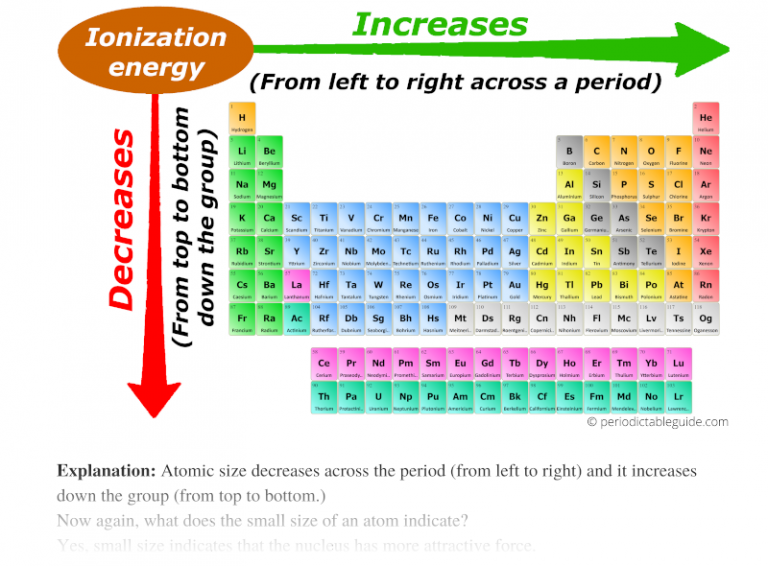
The periodic table, a cornerstone of chemistry, provides a systematic arrangement of elements based on their atomic number and recurring chemical properties. Within this framework, several ionic trends emerge, dictating how elements behave when forming ionic bonds. These trends are crucial for understanding the reactivity of elements, the formation of compounds, and the properties of materials.
Understanding Ionic Trends
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. Cations, positively charged ions, are formed when an atom loses electrons, while anions, negatively charged ions, are formed when an atom gains electrons. The strength and stability of ionic bonds are influenced by several factors, including:
- Ionization Energy: The energy required to remove an electron from a gaseous atom. Lower ionization energies indicate a greater tendency to form cations.
- Electron Affinity: The energy change that occurs when an electron is added to a gaseous atom. Higher electron affinities indicate a greater tendency to form anions.
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. Higher electronegativity values indicate a stronger attraction for electrons, making an element more likely to form anions.
- Ionic Radius: The size of an ion. Smaller ionic radii generally lead to stronger ionic bonds due to closer proximity of charges.
Key Trends in the Periodic Table
1. Ionization Energy:
- Increases across a period: As you move from left to right across a period, the atomic number increases, leading to a greater nuclear charge. This stronger attraction between the nucleus and electrons makes it more difficult to remove an electron, resulting in higher ionization energies.
- Decreases down a group: As you move down a group, the number of electron shells increases, placing the outermost electrons further from the nucleus. This weaker attraction between the nucleus and valence electrons makes it easier to remove an electron, resulting in lower ionization energies.
2. Electron Affinity:
- Generally increases across a period: As you move across a period, the increasing nuclear charge attracts electrons more strongly, leading to a greater energy release upon adding an electron. However, there are exceptions to this trend, particularly for elements with half-filled or fully filled electron shells.
- Decreases down a group: As you move down a group, the increasing distance between the nucleus and the added electron weakens the attraction, resulting in lower electron affinities.
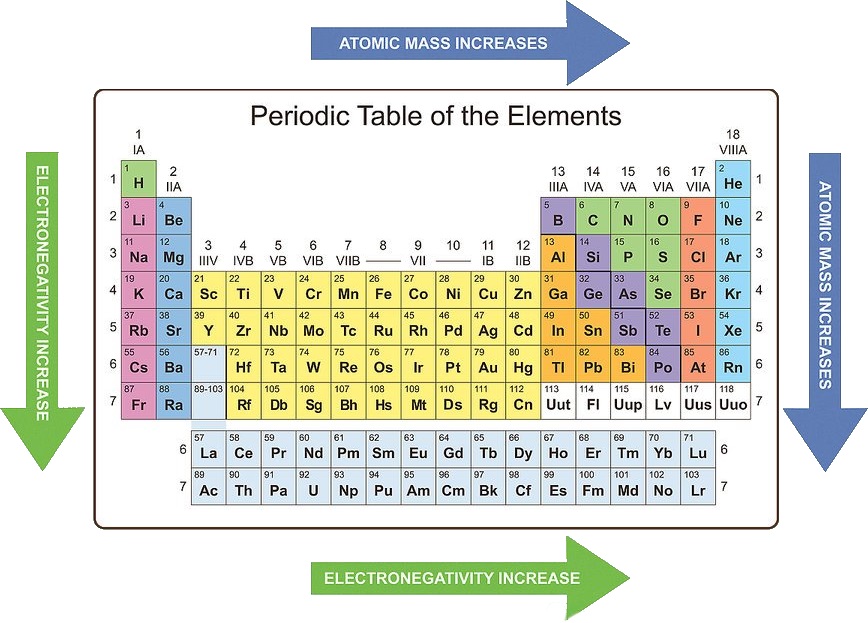
3. Electronegativity:
- Increases across a period: As you move across a period, the increasing nuclear charge attracts electrons more strongly, leading to higher electronegativity values.
- Decreases down a group: As you move down a group, the increasing distance between the nucleus and valence electrons weakens the attraction, resulting in lower electronegativity values.
4. Ionic Radius:
- Decreases across a period: As you move across a period, the increasing nuclear charge pulls the electrons closer to the nucleus, resulting in smaller ionic radii.
- Increases down a group: As you move down a group, the increasing number of electron shells leads to larger ionic radii.
Applications of Ionic Trends
Understanding these trends has significant implications in various fields:
- Predicting Chemical Reactions: Ionic trends help predict the likelihood of a reaction occurring and the type of compounds that will form. For example, elements with high electronegativity values are more likely to form ionic bonds with elements with low electronegativity values.
- Designing Materials: Ionic trends play a vital role in materials science. By understanding the ionic properties of elements, scientists can design materials with specific properties, such as conductivity, strength, or reactivity.
- Understanding Biological Processes: Ionic interactions are essential for biological processes, including the function of enzymes, the transmission of nerve impulses, and the stability of DNA.
- Environmental Chemistry: Ionic trends are crucial for understanding the fate and transport of pollutants in the environment.
Related Searches
1. Periodic Trends in Atomic Radius: This topic explores how the size of atoms changes across the periodic table, impacting various chemical properties.
2. Periodic Trends in Metallic Character: This topic investigates how the tendency of an element to lose electrons and exhibit metallic properties varies across the periodic table.
3. Periodic Trends in Nonmetallic Character: This topic examines how the tendency of an element to gain electrons and exhibit nonmetallic properties varies across the periodic table.
4. Periodic Trends in Reactivity: This topic discusses how the reactivity of elements changes across the periodic table, impacting their tendency to participate in chemical reactions.
5. Periodic Trends in Oxidation States: This topic explores how the possible charges an atom can take in ionic compounds vary across the periodic table.
6. Periodic Trends in Melting Point: This topic investigates how the temperature at which a substance transitions from a solid to a liquid varies across the periodic table, influenced by factors like ionic bonding strength.
7. Periodic Trends in Boiling Point: This topic examines how the temperature at which a substance transitions from a liquid to a gas varies across the periodic table, also influenced by ionic bonding strength.
8. Periodic Trends in Electronegativity Difference: This topic focuses on the difference in electronegativity between two atoms in a bond, which determines the type of bond formed (ionic, covalent, or polar covalent).
FAQs
Q: How do ionic trends relate to the formation of ionic compounds?
A: Ionic trends directly influence the formation of ionic compounds. Elements with a high tendency to lose electrons (low ionization energy) will readily form cations, while elements with a high tendency to gain electrons (high electron affinity) will readily form anions. The electrostatic attraction between these oppositely charged ions leads to the formation of ionic compounds.
Q: How do ionic trends affect the properties of ionic compounds?
A: Ionic trends significantly influence the properties of ionic compounds. For example, compounds formed by elements with small ionic radii and high charges tend to have higher melting and boiling points due to stronger electrostatic attractions. Additionally, the solubility of ionic compounds in water is influenced by the strength of the ionic bond and the interactions between the ions and water molecules.
Q: Are there any exceptions to the ionic trends?
A: While the periodic trends are generally observed, exceptions can occur. For example, the electron affinity of elements with half-filled or fully filled electron shells can deviate from the expected trend. Additionally, the size of ions can be influenced by factors like electron configuration and shielding effects.
Q: How can I use ionic trends to predict the reactivity of elements?
A: Ionic trends provide a framework for predicting the reactivity of elements. Elements with low ionization energies and high electronegativity values tend to be more reactive, readily forming ionic bonds with other elements. Conversely, elements with high ionization energies and low electronegativity values tend to be less reactive.
Tips for Understanding Ionic Trends
- Visualize the periodic table: Use a periodic table with clearly labeled periods and groups to visualize the trends.
- Focus on key factors: Pay attention to ionization energy, electron affinity, electronegativity, and ionic radius.
- Practice with examples: Work through examples of different elements and their properties to solidify your understanding.
- Connect the trends to chemical reactions: Understand how ionic trends influence the formation of compounds and the reactivity of elements.
Conclusion
The ionic trends in the periodic table provide a powerful framework for understanding the behavior of elements and predicting their reactivity. These trends are essential for chemists, material scientists, and researchers in various fields. By understanding the factors that influence ionic properties, we can gain valuable insights into the formation of compounds, the design of materials, and the mechanisms of chemical reactions. Mastering these trends opens doors to a deeper understanding of the fascinating world of chemistry.

/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)



Closure
Thus, we hope this article has provided valuable insights into Navigating the Periodic Table: Understanding Trends in Ionic Properties. We hope you find this article informative and beneficial. See you in our next article!
.PNG)