Understanding the Periodic Trends: A Deep Dive into Ionization Energy
Related Articles: Understanding the Periodic Trends: A Deep Dive into Ionization Energy
Introduction
With great pleasure, we will explore the intriguing topic related to Understanding the Periodic Trends: A Deep Dive into Ionization Energy. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding the Periodic Trends: A Deep Dive into Ionization Energy
The periodic table is a fundamental tool in chemistry, organizing elements based on their atomic structure and predictable properties. One of these key properties is ionization energy, which refers to the minimum energy required to remove an electron from a gaseous atom in its ground state. Understanding ionization energy trends allows us to predict the reactivity and behavior of elements, paving the way for advancements in various fields, including materials science, medicine, and technology.
Ionization energy trends are directly related to the arrangement of electrons within an atom, specifically the outermost valence electrons. These electrons experience a combination of attractive forces from the positively charged nucleus and repulsive forces from other electrons in the atom. This delicate balance dictates the ease with which an electron can be removed.
Factors Influencing Ionization Energy:
- Nuclear Charge: A higher nuclear charge (more protons) exerts a stronger attractive force on electrons, making it more difficult to remove them. This explains why ionization energy generally increases across a period.
- Atomic Radius: A smaller atomic radius signifies a stronger attraction between the nucleus and valence electrons, resulting in higher ionization energy. This is also observed across a period, as atoms become smaller due to increased nuclear charge.
- Shielding Effect: Inner electrons shield valence electrons from the full nuclear charge. As the number of inner electrons increases down a group, the shielding effect becomes more pronounced, leading to a weaker attraction between the nucleus and valence electrons, and consequently lower ionization energy.
- Electron Configuration: Elements with full or half-filled electron shells exhibit greater stability, requiring more energy to remove an electron. This explains the slight irregularities observed in ionization energy trends for certain elements.
Trends Across the Periodic Table:
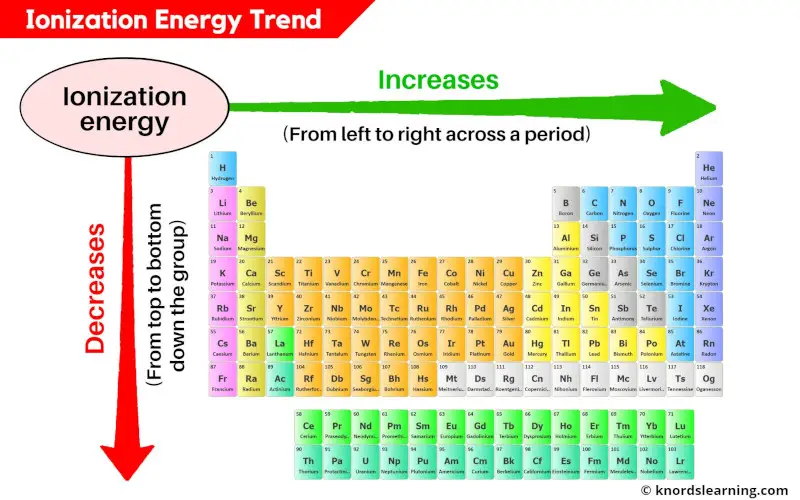
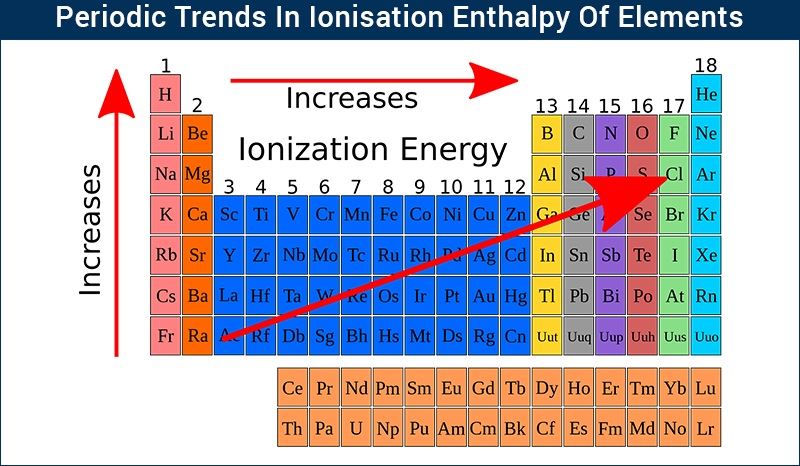
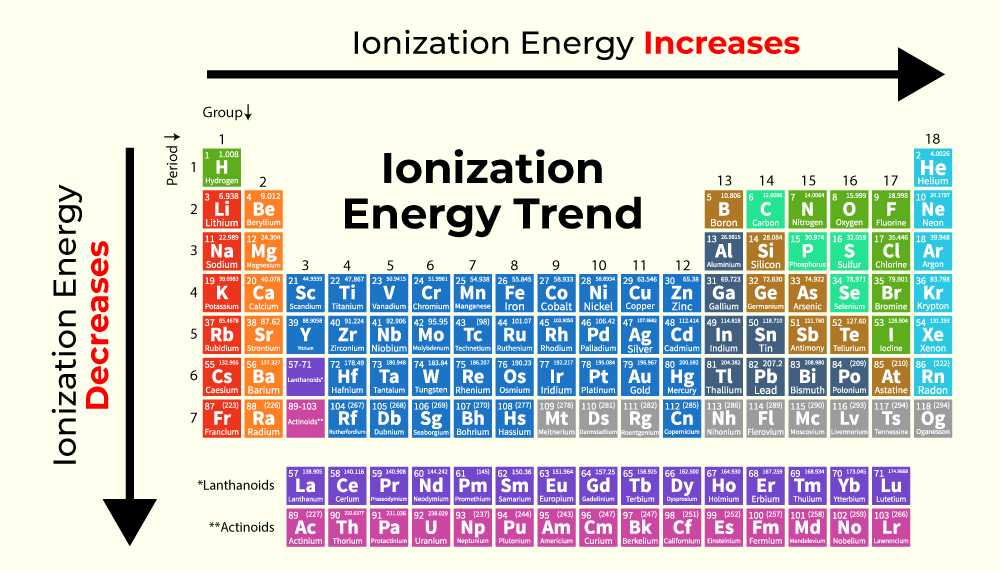
- Across a Period: Ionization energy generally increases from left to right across a period. This is due to increasing nuclear charge and decreasing atomic radius, leading to a stronger attraction between the nucleus and valence electrons.
- Down a Group: Ionization energy generally decreases from top to bottom down a group. This is primarily attributed to the increasing atomic radius and shielding effect, resulting in a weaker attraction between the nucleus and valence electrons.
Exceptions to the Trends:
While the general trends hold true for most elements, there are notable exceptions:
- Group 3 Elements (Sc, Y, La): These elements have slightly higher ionization energies than expected due to the presence of a single electron in the outermost d-orbital.
- Group 6 Elements (O, S, Se, Te): These elements exhibit a slight decrease in ionization energy between the second and third periods due to the increased electron-electron repulsion in the smaller oxygen atom.
- Group 15 Elements (N, P, As, Sb): Similar to Group 6 elements, a slight decrease in ionization energy is observed between the second and third periods due to electron-electron repulsion.
Applications and Importance:
Understanding ionization energy trends is crucial for various scientific and technological advancements:
- Predicting Chemical Reactivity: Elements with low ionization energies tend to readily lose electrons, making them highly reactive. This knowledge is essential in predicting chemical reactions and designing new materials.
- Materials Science: Ionization energy plays a vital role in the development of new materials with specific properties. For instance, understanding the ionization energy of different elements allows scientists to design semiconductors with specific band gaps for electronic devices.
- Medicine: Ionization energy is crucial in understanding the mechanisms of drug action and the development of new therapeutic agents. For example, the ionization energy of metal ions can influence their interaction with biological molecules and their effectiveness as drugs.
- Spectroscopy: Ionization energy data is essential for interpreting spectroscopic data, providing insights into the electronic structure of atoms and molecules.
Further Exploration:
- Successive Ionization Energies: Each atom can lose multiple electrons, and the energy required to remove each subsequent electron is known as the successive ionization energy. These values generally increase as more electrons are removed, reflecting the increasing difficulty of removing electrons from a positively charged ion.
- Electron Affinity: Closely related to ionization energy is electron affinity, which refers to the energy change when an electron is added to a neutral atom. Elements with high ionization energies generally have low electron affinities, indicating a preference for losing electrons rather than gaining them.
Related Searches:
- Periodic Trends: This broad search encompasses all the major trends observed in the periodic table, including ionization energy, electronegativity, atomic radius, and electron affinity.
- Electronegativity Trends: Electronegativity measures an atom’s ability to attract electrons in a chemical bond. It follows similar trends to ionization energy, with electronegativity increasing across a period and decreasing down a group.
- Atomic Radius Trends: Atomic radius is a measure of the size of an atom. It generally decreases across a period and increases down a group, reflecting the balance between nuclear charge and electron shielding.
- Electron Affinity Trends: Electron affinity measures the energy change when an electron is added to a neutral atom. It generally increases across a period and decreases down a group, though with more exceptions than ionization energy trends.
- Chemical Reactivity: Ionization energy is a key factor in determining an element’s chemical reactivity. Elements with low ionization energies tend to be highly reactive, readily losing electrons to form positive ions.
- Metallic Character: Metallic character refers to the tendency of an element to lose electrons and form positive ions. It generally increases down a group and decreases across a period, mirroring ionization energy trends.
- Non-Metallic Character: Non-metallic character refers to the tendency of an element to gain electrons and form negative ions. It generally decreases down a group and increases across a period, opposing ionization energy trends.
- Periodic Table Groups: Each group in the periodic table contains elements with similar chemical properties due to their similar valence electron configurations. Understanding ionization energy trends within each group helps predict the behavior and reactivity of elements within that group.
FAQs:
- What is the significance of ionization energy in chemistry? Ionization energy is a fundamental property that determines an atom’s tendency to lose electrons and form ions. This knowledge is crucial for predicting chemical reactivity, understanding chemical bonding, and interpreting spectroscopic data.
- How does ionization energy relate to electronegativity? Both ionization energy and electronegativity measure an atom’s ability to attract electrons. Elements with high ionization energies generally have low electronegativities, indicating a preference for losing electrons rather than gaining them.
- What are the exceptions to ionization energy trends? While the general trends of increasing ionization energy across a period and decreasing down a group hold true for most elements, there are exceptions due to factors such as electron configuration, electron-electron repulsion, and the presence of d-orbitals.
- How can I use ionization energy to predict chemical reactivity? Elements with low ionization energies readily lose electrons, making them highly reactive. Elements with high ionization energies tend to be less reactive, as they require more energy to lose electrons.
- What are some applications of ionization energy in different fields? Ionization energy is essential in various fields, including materials science (designing semiconductors), medicine (understanding drug action), and spectroscopy (interpreting spectroscopic data).
Tips for Understanding Ionization Energy Trends:
- Focus on the Valence Electrons: Ionization energy primarily concerns the removal of valence electrons, the outermost electrons in an atom. Pay attention to the number and arrangement of valence electrons.
- Consider Nuclear Charge and Atomic Radius: A higher nuclear charge and smaller atomic radius result in a stronger attraction between the nucleus and valence electrons, leading to higher ionization energy.
- Remember the Shielding Effect: Inner electrons shield valence electrons from the full nuclear charge, reducing the attraction between the nucleus and valence electrons.
- Recognize Exceptions: Be aware of the exceptions to the general trends, such as the slight decrease in ionization energy for Group 6 and 15 elements between the second and third periods.
- Relate Ionization Energy to Other Periodic Trends: Understand how ionization energy relates to other periodic trends, such as electronegativity, atomic radius, and electron affinity.
Conclusion:
Ionization energy trends are a fundamental aspect of chemistry, providing a framework for understanding the reactivity, bonding, and electronic structure of elements. By comprehending these trends, we gain valuable insights into the behavior of atoms and molecules, paving the way for advancements in various fields, including materials science, medicine, and technology. As we continue to explore the intricacies of the periodic table, understanding ionization energy trends will remain crucial for unlocking the secrets of the chemical world.
.PNG)




Closure
Thus, we hope this article has provided valuable insights into Understanding the Periodic Trends: A Deep Dive into Ionization Energy. We appreciate your attention to our article. See you in our next article!
