Unraveling the Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
Related Articles: Unraveling the Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Unraveling the Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Unraveling the Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
- 2 Introduction
- 3 Unraveling the Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
- 3.1 Delving into the Key Periodic Trends
- 3.2 Understanding the Significance of Periodic Trends
- 3.3 Exploring Related Searches and FAQs
- 3.4 Tips for Mastering Periodic Trends
- 3.5 Conclusion
- 4 Closure
Unraveling the Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior
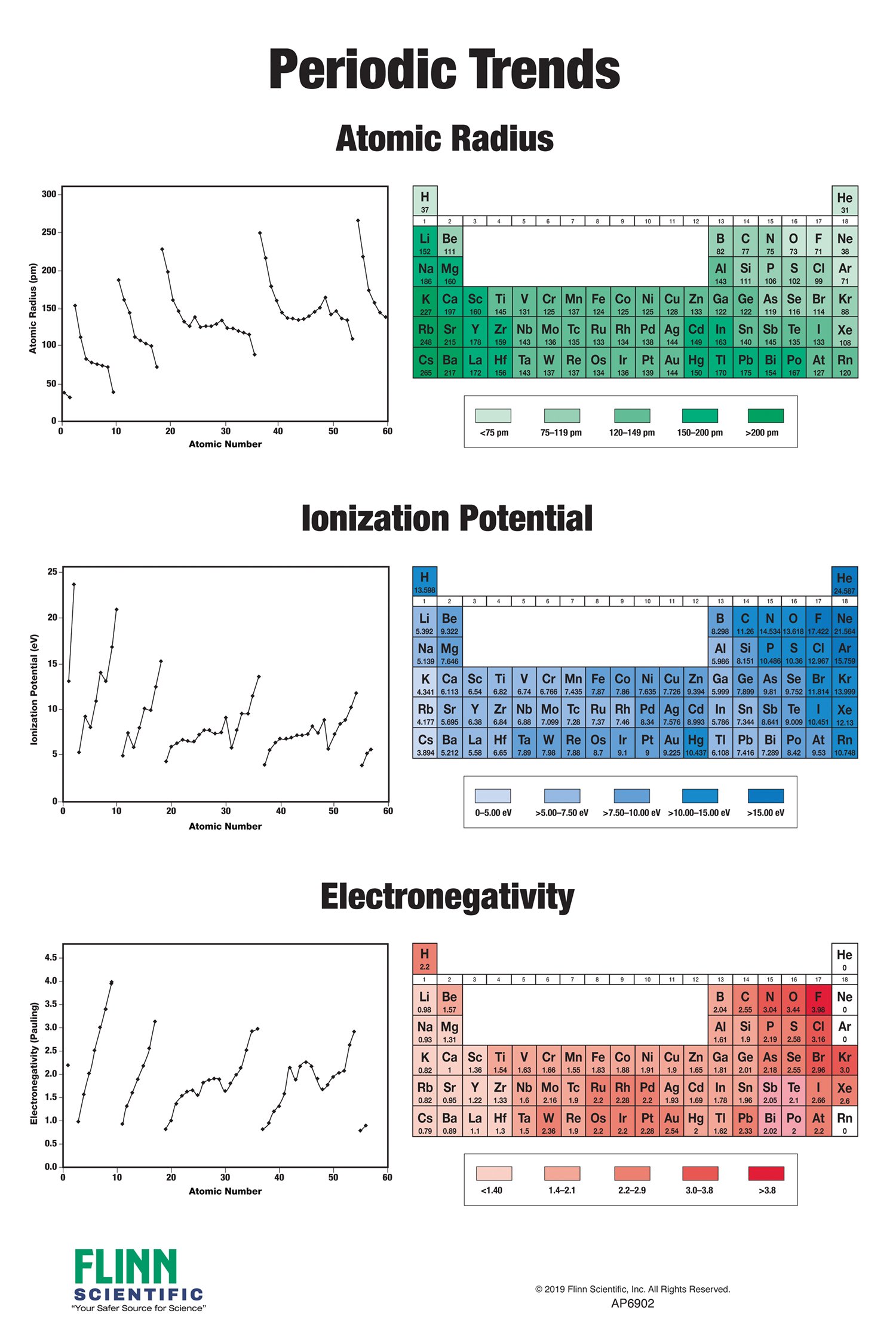
The periodic table, a cornerstone of chemistry, provides a systematic organization of elements based on their atomic structure and recurring chemical properties. These recurring patterns, known as periodic trends, are essential for understanding the behavior of elements and predicting their reactivity.
Periodic Trends Gizmo Answers 2025 is a valuable tool for exploring these trends in a dynamic and interactive manner. It allows students to visualize and manipulate the periodic table, observing how various properties change as you move across periods and down groups. This article will delve into the intricacies of periodic trends and their significance, utilizing the insights gleaned from Periodic Trends Gizmo Answers 2025 as a springboard for deeper understanding.
Delving into the Key Periodic Trends
Periodic Trends Gizmo Answers 2025 highlights the following key trends:
- Atomic Radius: This trend describes the size of an atom, measured as the distance from the nucleus to the outermost electron shell. As you move across a period from left to right, the atomic radius decreases due to increasing nuclear charge and a stronger attraction between the nucleus and electrons. Conversely, moving down a group, the atomic radius increases as the number of electron shells increases.
- Ionization Energy: Ionization energy is the minimum energy required to remove an electron from a gaseous atom in its ground electronic state. This trend follows a similar pattern to atomic radius. Ionization energy increases across a period due to the increasing nuclear charge and stronger attraction for electrons, making it harder to remove an electron. However, it decreases down a group as the outermost electron is further from the nucleus and experiences less attraction.
- Electron Affinity: Electron affinity is the change in energy when an electron is added to a neutral atom to form a negative ion. While it generally increases across a period, there are exceptions due to electron configuration and electron-electron repulsion. Down a group, electron affinity tends to decrease as the added electron is further from the nucleus and experiences less attraction.
- Electronegativity: Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. It increases across a period as the nuclear charge increases and the electrons are held more tightly. Down a group, electronegativity decreases as the outermost electron is further from the nucleus and less attracted.
- Metallic Character: Metallic character refers to the tendency of an element to lose electrons and form positive ions. It increases down a group as the ionization energy decreases, making it easier for the atom to lose electrons. Conversely, it decreases across a period as the ionization energy increases.
Understanding the Significance of Periodic Trends
The Periodic Trends Gizmo Answers 2025 provides a platform to visualize these trends and their implications. By manipulating the interactive periodic table, users can directly observe how these trends influence:
- Chemical Reactivity: Elements with low ionization energies and high metallic character tend to be more reactive, readily losing electrons to form cations. Conversely, elements with high ionization energies and high electronegativity are less reactive, tending to gain electrons and form anions.
- Bond Formation: Electronegativity differences between atoms determine the type of bond formed. Large differences lead to ionic bonds, where one atom loses an electron and the other gains it. Smaller differences result in covalent bonds, where electrons are shared between atoms.
- Physical Properties: Trends in atomic radius, ionization energy, and electronegativity influence various physical properties like melting point, boiling point, and conductivity. For example, metals with low ionization energies tend to have high melting points due to strong metallic bonding.
Exploring Related Searches and FAQs
Periodic Trends Gizmo Answers 2025 is a powerful tool that encourages further exploration of related concepts. Here are some common searches and FAQs that arise:
Related Searches:
- Periodic Table Trends Explained: This search explores the underlying reasons behind the observed trends, delving into the atomic structure and electron configuration that influence these properties.
- Periodic Trends and Chemical Bonding: This search investigates how periodic trends influence the types of bonds formed between elements, emphasizing the role of electronegativity differences.
- Periodic Trends and Physical Properties: This search examines the correlation between periodic trends and various physical properties, such as melting point, boiling point, and conductivity.
- Exceptions to Periodic Trends: This search investigates the exceptions to the general trends, exploring the reasons behind these deviations and their impact on chemical behavior.
- Periodic Trends Gizmo Activities: This search leads to interactive activities and exercises designed to solidify understanding of periodic trends using the Periodic Trends Gizmo Answers 2025.
- Periodic Trends and the Chemistry of Life: This search explores the role of periodic trends in understanding the chemistry of essential elements in living organisms, such as carbon, oxygen, and nitrogen.
- Periodic Trends and Modern Chemistry: This search investigates the application of periodic trends in contemporary chemical research and technological advancements, highlighting their relevance in fields like materials science and nanotechnology.
FAQs:
- Why do atomic radii decrease across a period? This is due to increasing nuclear charge, which attracts electrons more strongly, pulling them closer to the nucleus.
- Why do ionization energies increase across a period? The same reason as above – stronger attraction between the nucleus and electrons makes it harder to remove an electron.
- What is the relationship between ionization energy and metallic character? Elements with low ionization energies have high metallic character because they readily lose electrons.
- How do periodic trends affect chemical reactivity? Elements with low ionization energies and high metallic character are more reactive, while those with high ionization energies and high electronegativity are less reactive.
- What are some exceptions to periodic trends? Some exceptions occur due to electron configuration and electron-electron repulsion, particularly in the case of electron affinity.
Tips for Mastering Periodic Trends
Periodic Trends Gizmo Answers 2025 provides an interactive platform for mastering these concepts. Here are some tips to maximize your learning:
- Visualize the Trends: Use the interactive periodic table to observe how properties change across periods and down groups.
- Relate Trends to Atomic Structure: Connect the observed trends to the underlying atomic structure, including the number of protons, neutrons, and electrons.
- Apply Trends to Predict Reactivity: Use the trends to predict the reactivity of elements and the types of bonds they form.
- Explore Exceptions: Understand the reasons behind exceptions to the general trends and their impact on chemical behavior.
- Practice with Examples: Use the Periodic Trends Gizmo Answers 2025 to solve problems and apply your knowledge to real-world scenarios.
Conclusion
Periodic Trends Gizmo Answers 2025 is a valuable tool for visualizing and understanding the fundamental principles of chemistry. By exploring these trends, we gain insights into the reactivity, bonding, and physical properties of elements, laying the foundation for further exploration in the vast world of chemistry. The periodic table, with its organized arrangement of elements and their properties, serves as a powerful map guiding our understanding of chemical behavior and its implications in various fields of science and technology.
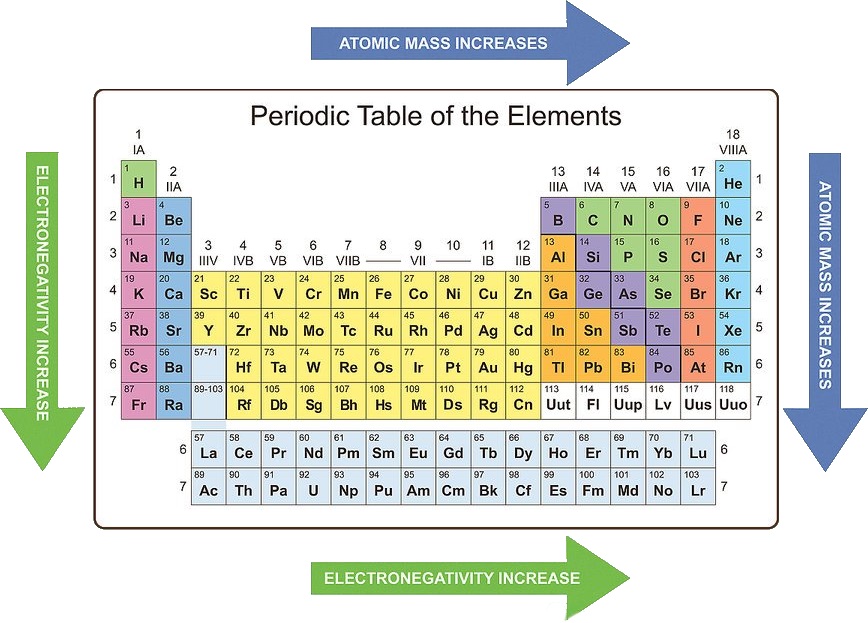



/periodictrendstable-5c4a46614cedfd000187c5db.jpg)

Closure
Thus, we hope this article has provided valuable insights into Unraveling the Periodic Trends: A Comprehensive Guide to Understanding Chemical Behavior. We hope you find this article informative and beneficial. See you in our next article!
.PNG)